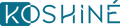KX-826 vs. Common Hair Loss Solutions
|
Category |
KX-826 |
Minoxidil |
Finasteride (Oral/Topical) |
|
Mechanism of Action |
Clear mechanism, topical use. |
Unclear mechanism, topical use. |
Clear mechanism, oral or topical use. |
|
Impact on Hormone Levels |
No effect on systemic hormone levels. |
No direct effect. |
Significantly reduces DHT, potentially causing hormonal imbalance. |
|
Systemic Absorption |
Extremely low; clinical studies confirm good safety. |
Certain systemic absorption; may affect pet health. |
Fully absorbed orally; topical use also poses systemic absorption risks. |
|
Dosage and Usage |
Twice daily. |
Twice daily. |
Once daily, one tablet per use. |
|
Target Users |
Suitable for both men and women (depending on indications). |
Suitable for both men and women (but limited efficacy in women). |
Primarily for men. |
|
Common Side Effects |
Low incidence of adverse events; good safety profile. |
Minoxidil use is associated with a pronounced shedding phase at the beginning of treatment, characterized by an initial worsening of hair loss and increased scalp greasiness. It may also induce hypertrichosis, resulting in unwanted hair growth on the face, neck, or other non-target areas of the body. Furthermore, minoxidil is highly toxic to pets, particularly cats and dogs, with multiple reported cases of poisoning and even fatalities due to accidental exposure. Therefore, it is crucial to take stringent precautions to prevent any contact with pets and ensure their safety during use. |
Severe systemic side effects, including sexual dysfunction, emotional changes, fatigue, and depression. |
|
User Experience (Convenience & Portability) |
Cosmetic product. |
|
Prescription oral drug. |
|
Clinical Efficacy |
In a Phase II clinical trial in Chinese men with androgenetic alopecia, after 24 weeks of using KX-826 at 0.5% concentration, an average of approximately 22.73 new hairs per cm² were observed, over 15 more hairs than the placebo group. The difference was highly statistically significant (P<0.001), showing a remarkable and visible improvement. |
According to clinical studies conducted by 3SBio, after 24 weeks of treatment with 5% minoxidil foam, efficacy was comparable to the internationally renowned brand ROGAINE®, with similar safety and tolerability. However, a shedding phase is common during the early stages of treatment, requiring continued use and patience. |
Cutia is advancing the marketing of CU-40102 (topical Finasteride spray). In their disclosed Phase III trial in China, after 24 weeks of treatment, both total hair count and terminal hair count in the target area significantly increased compared to the placebo (P<0.05). |
|
Product Positioning |
A new generation of topical AR antagonists. |
Traditional method of improving blood circulation. |
Hormone-regulating drug with significant safety controversies. |